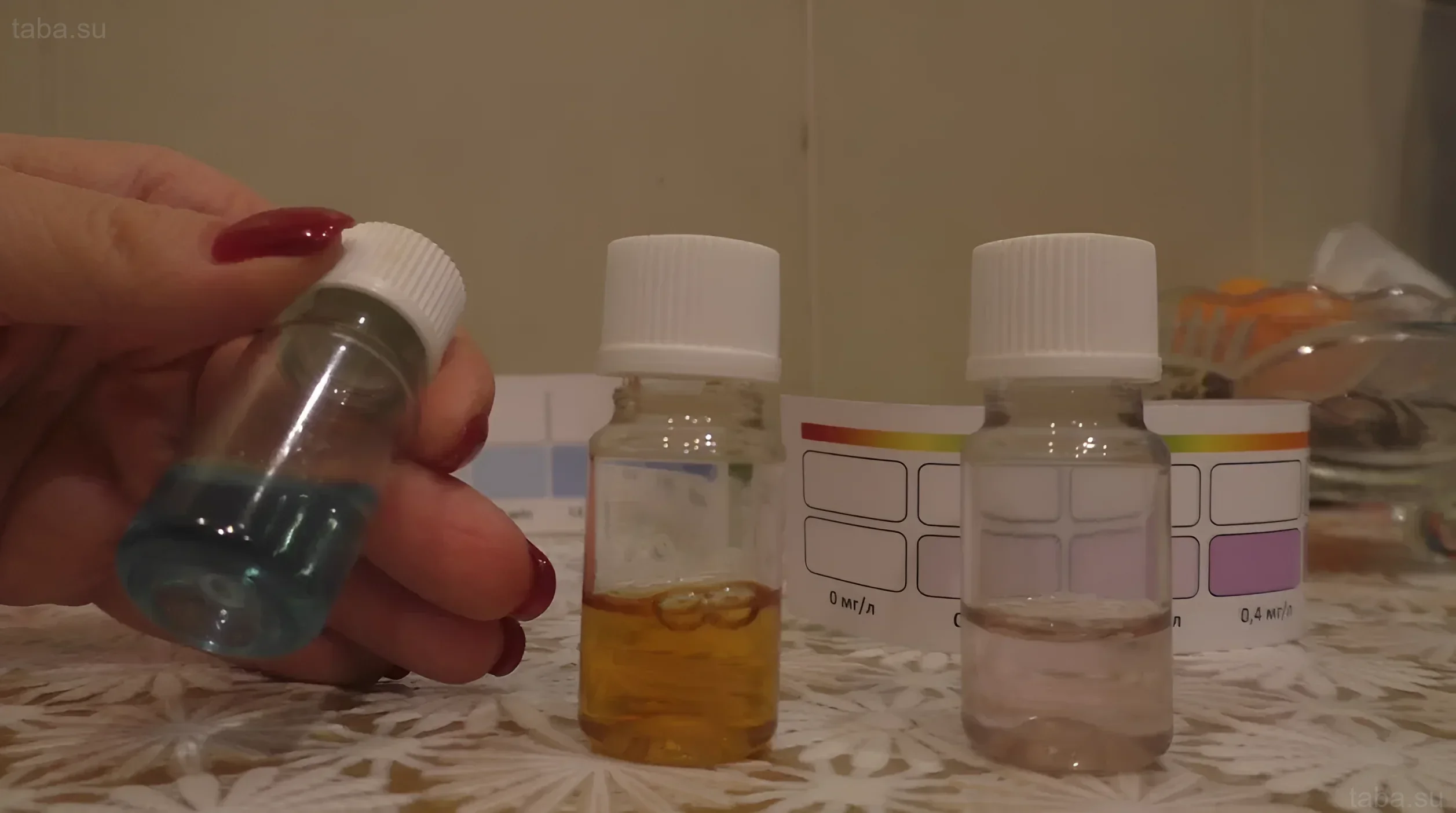Maintaining optimal water parameters is the cornerstone of successful aquaristics. Among all chemical indicators, pH, or the hydrogen potential, holds a central place. It is pH that determines how comfortable and safe the environment will be for aquarium inhabitants, affecting their metabolism, immunity, and reproductive capacity.
For the leading experts of the Taba.su portal, pH is not just a number; it is a critically important indicator of the ecosystem’s health. Sharp fluctuations or prolonged deviation from the required level can cause stress, weaken immunity, and even lead to the death of sensitive species. Understanding what pH is, how it changes, and how to manage it is a mandatory skill for every aquarist.
What is pH and How is it Measured? Chemistry Basics for the Aquarist

pH (from Latin potentia hydrogenii – “power of hydrogen”) is an indicator of the concentration of hydrogen ions (H+) in an aqueous solution. Simply put, it determines the acidity or alkalinity of the water.
The pH scale is logarithmic and ranges from 0 to 14:
- pH 7.0: Neutral water. The concentration of H+ and OH- (hydroxide ions) is balanced.
- pH below 7.0: Acidic environment. Hydrogen ions (H+) dominate.
- pH above 7.0: Alkaline (basic) environment. Hydroxide ions (OH-) dominate.
Why is pH So Important for Fish?
Fish organisms are adapted to survive within a very narrow pH range characteristic of their natural habitat. Incorrect pH disrupts the process of osmoregulation – the fish’s ability to maintain the balance of salts and water in its body. Furthermore, when pH changes, the toxicity of ammonia (NH₃) sharply increases. In an alkaline environment (high pH), almost all ammonium (NH₄+) converts into deadly ammonia.
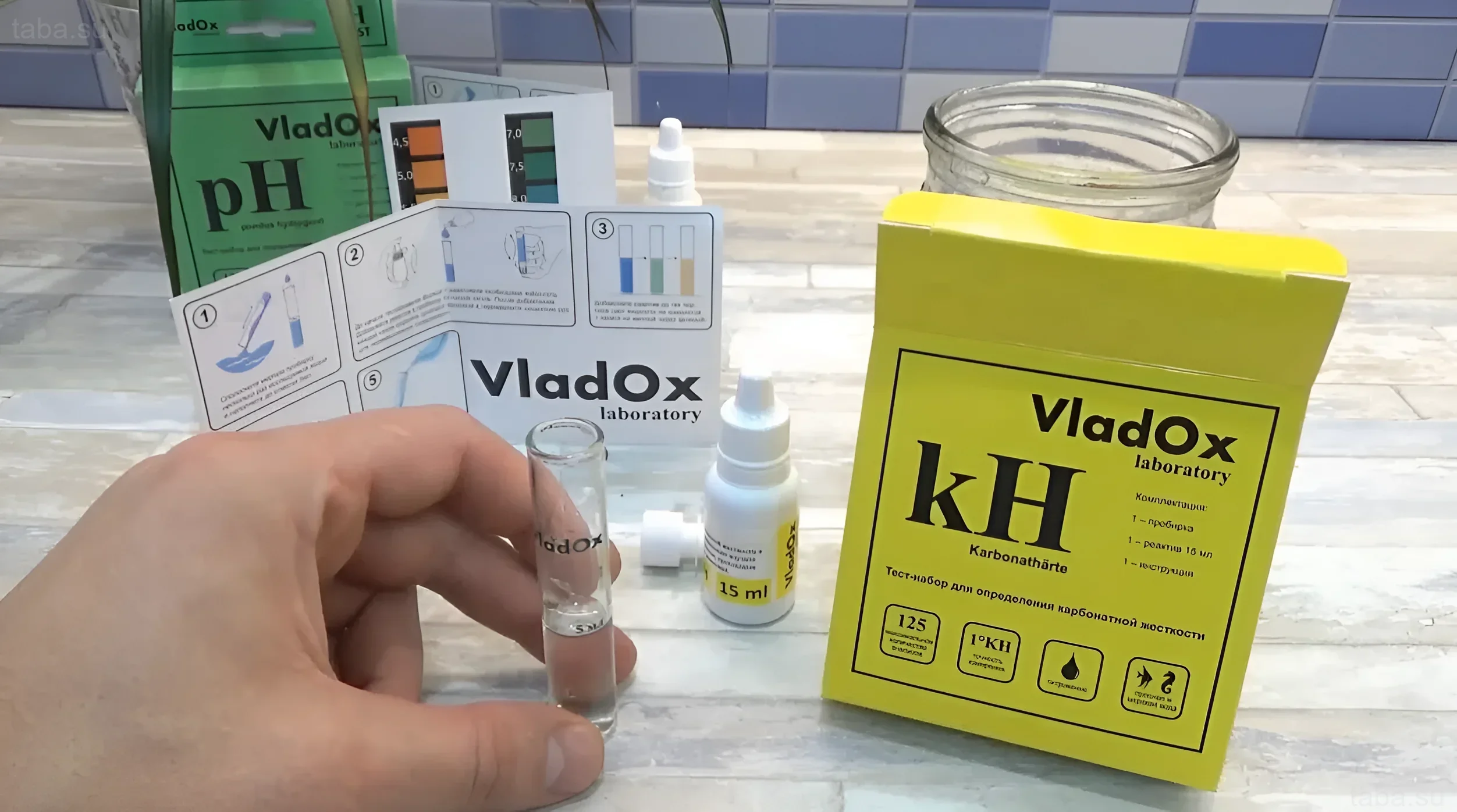
Methods of pH Measurement
Accurate and regular pH measurement is the key to stability. Experts recommend using the most reliable tools:
- Liquid Test Kits (Reagent Tests): The most common and accurate method for home use. They allow determining pH with an accuracy of 0.1–0.2 units.
- Electronic pH Meters: Provide high accuracy and instant results but require regular calibration and careful storage.
- Test Strips: Convenient for quick screening but less accurate than liquid tests and not recommended for continuous monitoring of critical parameters.
Ideal pH for Popular Aquarium Fish: Table and Recommendations
The choice of optimal pH directly depends on the species composition of the aquarium. Mixing fish that require extremely acidic and extremely alkaline water is a common beginner’s mistake.
Most tropical freshwater fish prefer slightly acidic to neutral water (pH 6.5–7.5).
| Environment Type | pH Range | Examples of Fish (with Latin names) |
|---|---|---|
| Acidic (Soft Water) | 5.5 – 6.5 | Discus (Symphysodon spp.), Neon Tetra (Paracheirodon innesi), Angelfish (Pterophyllum scalare), Apistogramma (Apistogramma spp.) |
| Neutral/Slightly Acidic | 6.5 – 7.5 | Guppy (Poecilia reticulata), Corydoras Catfish (Corydoras spp.), Tetra (Hyphessobrycon spp.), Rainbowfish (Melanotaenia spp.) |
| Alkaline (Hard Water) | 7.5 – 8.5 | Fish from Lake Malawi and Tanganyika (e.g., Frontosa Cichlid — Cyphotilapia frontosa), Livebearers (Mollies — Poecilia sphenops) |
Important: When choosing a pH, always consider the most sensitive species in your aquarium. If you keep Discus, a pH of 7.5, which is tolerable for Guppies, will be fatal for them.
Factors Affecting Aquarium pH: Causes of Fluctuations and How to Control Them
pH never remains static. It constantly fluctuates throughout the day and changes during the aquarium’s life processes. Experts identify key factors influencing acidity:
1. Buffering Capacity (KH)
This is perhaps the most important factor. Carbonate hardness (KH) is the water’s ability to resist changes in pH. The higher the KH, the more stable the pH. Substances containing carbonates and bicarbonates act as a “buffer,” neutralizing acids that form in the aquarium.
- Low KH: pH drops easily and quickly (so-called “pH crash”). Typical for aquariums using reverse osmosis water.
- High KH: pH is stable, but it is difficult to shift it in the desired direction (e.g., for breeding acid-loving species).
2. Carbon Dioxide (CO₂)
The dissolution of CO₂ in water leads to the formation of carbonic acid (H₂CO₃), which lowers pH. This process causes natural diurnal fluctuations:
- Night: Plants and fish release CO₂ during respiration. pH decreases.
- Day: Plants actively consume CO₂ for photosynthesis. pH increases.
3. Nitrification and Organic Acids
The process of nitrification (conversion of ammonia to nitrites, and then to nitrates, carried out by bacteria) produces nitric acid, which slowly but surely lowers pH. The accumulation of organic waste and humic acids (especially when using driftwood or peat) also contributes to a decrease in acidity.
4. External Materials
Some substrates and decorations can actively influence pH:
- Increase pH: Limestone, crushed marble, crushed coral. They contain carbonates that dissolve and increase buffering capacity and pH.
- Decrease pH: Special nutrient substrates for plants (e.g., baked earth), peat, natural driftwood.
How to Safely Change Aquarium pH: Step-by-Step Instructions and Warnings

The most important rule: pH changes should be slow and gradual. A sudden jump of even 0.3–0.4 units can cause pH shock.
Methods to Lower pH (for Acid-Loving Species)
The goal is to add acids or reduce KH.
- Using Peat: A natural and gentle method. Peat releases humic and fulvic acids, which lower pH and soften water. It can be placed in the filter or a peat extract can be used.
- CO₂ System: The most controlled method for lowering pH, especially in planted tanks. Requires precise control, as CO₂ overdose is dangerous for fish.
- Using Reverse Osmosis (RO) Water: Mixing tap water with reverse osmosis purified water (which has a pH of around 7.0 and KH=0) allows for controlled reduction of total hardness and buffering capacity, making the water more amenable to acidification.
- Chemical Buffers (pH Down): Use with caution. They can temporarily lower pH, but if KH is high, pH will quickly return to its original value. Recommended only for emergency situations.
Methods to Raise pH (for Alkaline-Loving Species)
The goal is to add carbonates and bicarbonates.
- Carbonate Substrates: Using crushed coral or marble in the filter or as substrate. This will provide a constant and stable increase in KH and pH.
- Baking Soda (Sodium Bicarbonate): A quick way to raise KH and pH. Should be used very sparingly and slowly, adding the solution to the water change. Primarily used for aquariums with African cichlids.
- Regular Water Changes: If the source tap water has a high pH and KH, regular water changes will naturally maintain an alkaline environment.
Problems Associated with Incorrect pH: Symptoms and Solutions

Incorrect pH is not just an inconvenience; it is a direct threat to the health of aquatic life. Problems can be caused by both too high and too low levels, as well as sharp fluctuations.
Symptoms of pH Stress in Fish
- Rapid breathing and shortness of breath: Fish stay at the surface, even though oxygen levels are normal. This can be a reaction to ammonia toxicity at high pH or gill dysfunction at low pH.
- Increased mucus production: A protective reaction of the body to gill irritation.
- Loss of color and apathy: General signs of severe stress.
- Behavioral changes: Erratic swimming, attempts to jump out of the water, or, conversely, complete lack of movement.
Problem: pH Crash
This is a sudden drop in pH (usually to 6.0 and below) that occurs in aquariums with very low buffering capacity (KH < 2 dKH). Acids formed during nitrification quickly deplete the buffer, and the pH crashes. This often happens after prolonged absence of water changes.
Solution: Immediately check KH. If KH is low, slowly and carefully add a sodium bicarbonate solution or perform a small water change with fresh tap water (if its KH is sufficiently high).
Expert Advice: Maintaining Stable Aquarium pH

Experienced aquarists know: stability is more important than perfection. It is better to keep fish at a stable pH of 7.8 than to let it fluctuate between 6.5 and 8.5.
Key Principles of Stability
- KH Control: Regularly test carbonate hardness. If you keep fish that require acidic water, maintain KH at a level of at least 3–4 dKH to prevent crashes. If KH constantly drops, it is a sign of insufficient buffering.
- Regular Changes: Weekly water changes (15–25%) with fresh water help remove accumulated nitrates and acids, and also restore buffering capacity.
- Inert Materials: Use only inert substrates and decorations if you aim for neutral or acidic pH (e.g., quartz sand, basalt, neutral stones).
- Aeration: Good aeration and water circulation help remove excess CO₂, which prevents excessive acidification, especially at night.
FAQ: Frequently Asked Questions About Aquarium pH

1. How often should pH be measured?
During the aquarium’s cycling and stabilization phase (the first 2–3 months), pH should be measured 2–3 times a week. In a mature aquarium, weekly measurement is sufficient, always before a water change.
2. Can plants affect pH?
Yes. During the day, when plants actively photosynthesize, they absorb CO₂, leading to an increase in pH. At night, during respiration, they release CO₂, leading to a decrease in pH. This diurnal cycle is usually not critical if KH is sufficiently high.
3. What is “Old Aquarium Syndrome”?
This is a situation where, in an aquarium that has not been maintained for a long time or has had insufficient water changes, the pH gradually drops to very low values (6.0 and below). This occurs due to the accumulation of organic acids and the depletion of buffering capacity. The solution is a gradual increase in KH and regular water changes.
4. Can vinegar be used to lower pH?
No, this is strictly not recommended. Vinegar (acetic acid) causes a sharp and uncontrolled drop in pH, which will inevitably lead to pH shock in fish. For safe pH reduction, peat, RO water, or specialized buffer solutions designed for aquaristics should be used.
Additional Images
Gallery of remaining images (click to view):